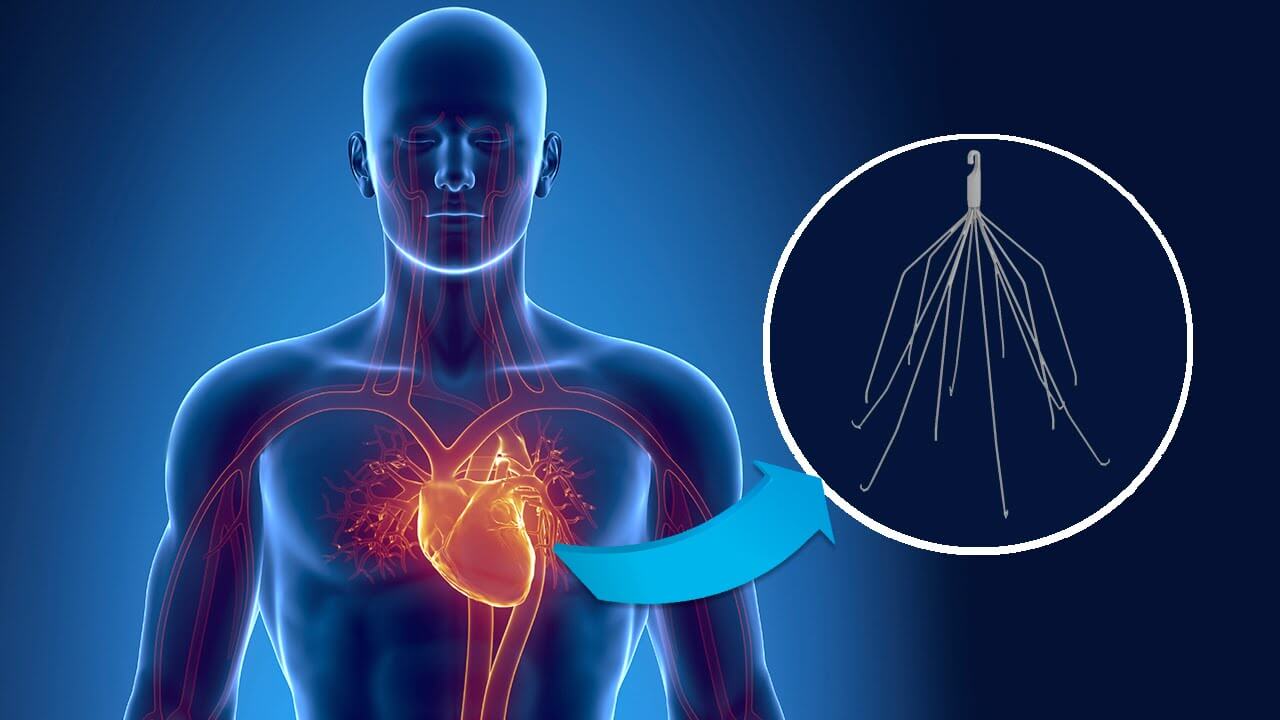
A South Dakota woman has filed a lawsuit against C.R. Bard after her Eclipse IVC filter failed, causing her health complications. The woman claims Bard failed to warn her of the risks associated with the Eclipse IVC filter.
The South Dakota woman had the Eclipse IVC filter implanted to catch any blood clots from her lower extremities before they traveled into her heart or lungs. Some time after implantation it was discovered the device had fractured, and several pieces had traveled throughout her body. Doctors have tried to remove the broken pieces, but were unsuccessful.
Bard’s first IVC filter was approved by the FDA in 2002. However, shortly after it was released onto the market, the company began to receive reports the devices were causing serious complications. In 2005, Bard voluntarily recalled its IVC filters from the market, and replaced them with a newer model, the G2 IVC filter. The Eclipse IVC filter is nearly identical to Bard’s G2 IVC filter, but the Eclipse has an electropolished finish that makes the device much smoother.
As reports of defective IVC filters continued, the FDA released a safety statement in 2010 stating the risk of an IVC filter failing greatly increases with time spent in the body. The FDA recommended removing the filter as soon as the threat of blood clots passed. The FDA updated its initial safety statement in 2014, recommending the devices be removed between 29 and 54 days after implantation.
But Bard isn’t the only one struggling with defective IVC filters. Cook Medical, Cordis Corporation, and Boston Scientific are all facing similar allegations. The first of these lawsuits go to trial against Cook Medical, who might be looking to settle hundreds of cases rather than go to trial. The resolution of Cook’s lawsuits will set the tone for Bard as it heads to court in early 2017.