Healthcare giant Johnson & Johnson (J&J) has been battling thousands of lawsuits against women who developed ovarian cancer from using the company’s baby powder products. However the company was dealt a monumental blow when plaintiffs’ were successful in obtaining the largest baby powder verdict to date. Jurors in Missouri determined J&J hid the cancer risks of baby powder from consumers and awarded 22 women $4.69 billion damages.

Johnson & Johnson Ordered To Pay $4.69 Billion To Talc Users For Causing Ovarian Cancer
Female Lawyer To Lead Class Action Over Essure Birth Control Device
Fidelma Fitzpatrick has been named lead counsel of the Bayer Essure California state court. Alameda County Superior Court Judge Winifred Smith appointed her to head a five-member plaintiffs executive committee in the Essure litigation last month. Smith’s order was made public on Jan. 6. About 50 lawsuits filed in California on behalf of more than 800 women allege Bayer failed to disclose its Essure contraceptive device could cause chronic pain, bleeding, and unintended pregnancies, among other side effects.
A man who suffered an adverse reaction after his hernia was patched with a polypropylene mesh product files suit this week in federal court claiming the product’s maker, Atrium Medical Corp. (Atrium), was negligent and hid the device’s dangers. Atrium was found in 1981 and delivers over 2.7 million medical products a year, one of which is its C-QUR polypropylene mesh with Omega 3 gel coating, more commonly known as fish oil.
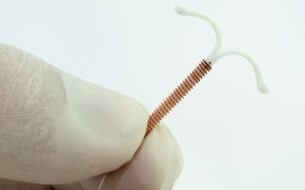
Plaintiffs Again Seek Consolidation of Mirena Pseudotumor Lawsuits
Mirena is an intrauterine device (IUD) inserted to prevent pregnancy. The Mirena birth control implant releases the prescription medication levonorgestrel. In addition to previous lawsuits filed over other side effects, Bayer now faces at least 116 Mirena Pseudotumor Cerebri lawsuits in 17 federal jurisdictions. Bayer not only makes Mirena, it also makes troubled contraceptive device Essure.
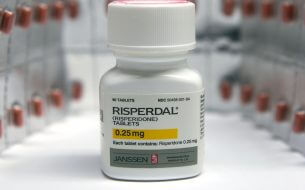
On the eve of a Philadelphia Risperdal trial, Johnson & Johnson and Janssen Pharmaceuticals Inc. agreed to settle a lawsuit alleging a New York boy grew female breasts after being treated with the antipsychotic medication. This case would have been the seventh trial over gynecomastia linked to the powerful antipsychotic.
Last year, healthcare giant Johnson & Johnson (J&J) lost three multimillion-dollar baby powder cancer trials. Not eager to jump into the next trial, the company asked the Missouri Court of Appeals to delay it. However, the company’s request was denied.

Morning Sickness Drug Called Into Question Over 50 Years Later
In the 1950s, the U.S. Food and Drug Administration (FDA) approved a combination of doxylamine and pyridoxine to treat morning sickness in pregnant women. Despite the long history in the pharmaceutical marketplace, some doctors are concerned the safety and efficacy of these drugs have never properly been established.
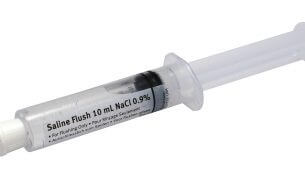
The U.S. Food and Drug Administration (FDA) has issued a Class I recall for over 380,000 saline flush syringes after discovering the syringes were possibly contaminated with a dangerous bacteria. Manufactured by Nurse Assist Inc., the Normal Saline Flush IV Syringes are contaminated with Burkholderia cepacia, a bacteria more commonly known as B. cepacia.
Healthcare giant Johnson & Johnson (J&J) and its subsidiary, DePuy Orthopedics, were ordered to pay six plaintiffs over $1 billion last month due to injuries caused by the defective Pinnacle hip implant. However, a Texas judge reduced the amount of damages earlier this week.
Atrium Medical Corporation recently came under fire over its hernia mesh products, and a number of lawsuits have been filed alleging its products are defective in design. In response to an overwhelming number of lawsuits, the United States Judicial Panel on Multidistrict Litigation consolidated all federal court lawsuits in the U.S. District Court for New Hampshire. The lawsuits are being presided over by Judge Landya B. McCafferty.