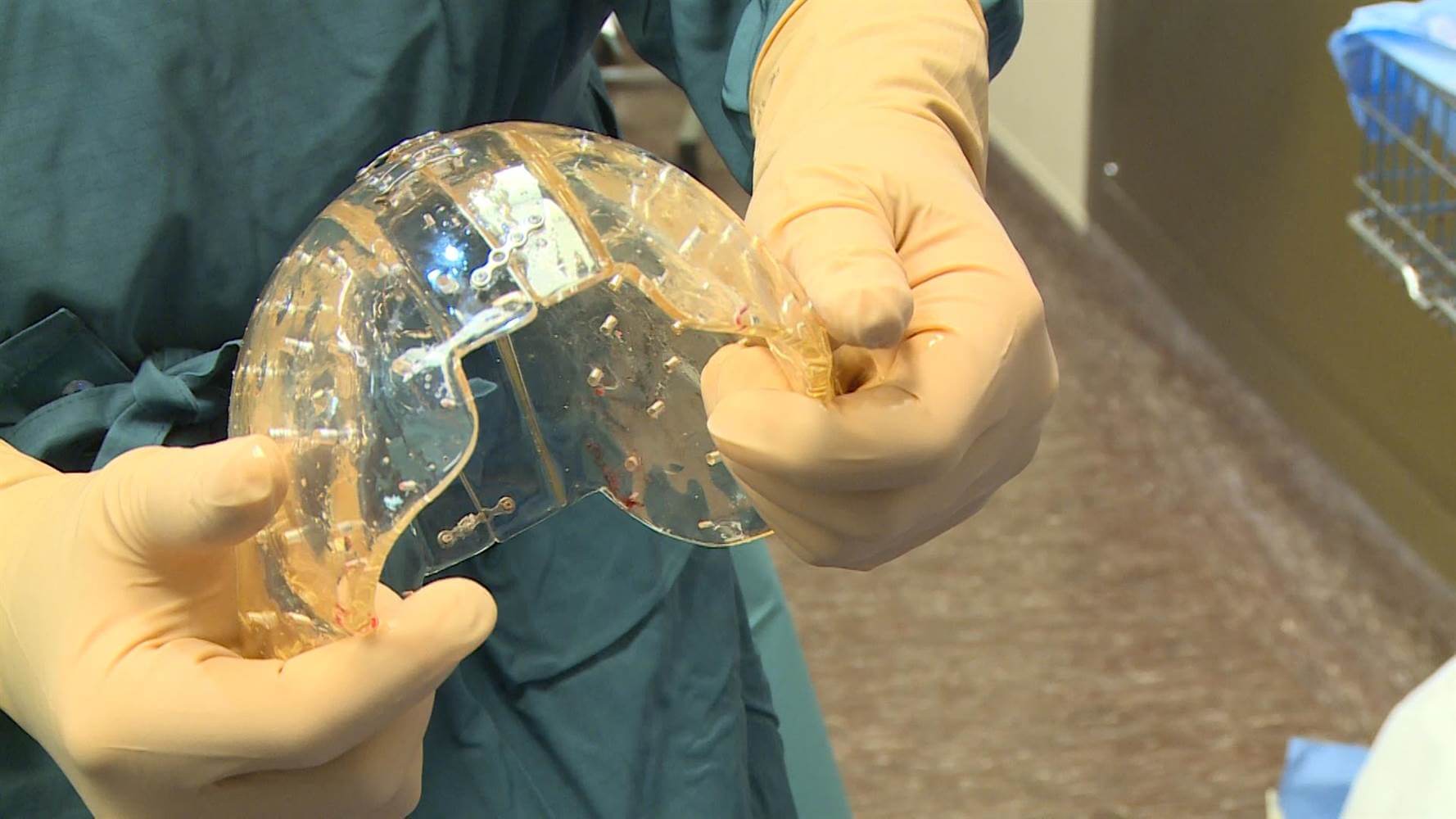
With 3D printing technology on the verge of taking the manufacturing industry by storm, several manufacturers are developing 3D printed medical devices. In response, the FDA has released guidelines to help manufacturers understand how the FDA will review the devices and what standards they need to meet.
The guidelines address issues such as materials, sanitation, how the devices will affect medical imaging, and performance and safety, among others. To read more of the FDA’s guidelines, you can view the document here.
Johnson & Johnson has partnered with an HP subsidiary to develop 3D printed medical products. The collaboration hopes to bring innovations to the healthcare industry that are not possible with existing standard manufacturing technologies.
However, 3D printed medical devices are not without their challenges. Because materials used in 3D printing are more porous than traditional devices, they are more difficult to sterilize. To date, the FDA has yet to approve any 3D printed medical devices that are considered high-risk, which leaves many wondering if 3D printed medical devices will be safe.
Devices such as Inferior Vena Cava Filters (IVC Filters) and the Essure birth control device have proven the FDA’s shortcomings in approving medical devices. With thousands of defective device victims across the country, is the FDA ready to take on another type of medical device?