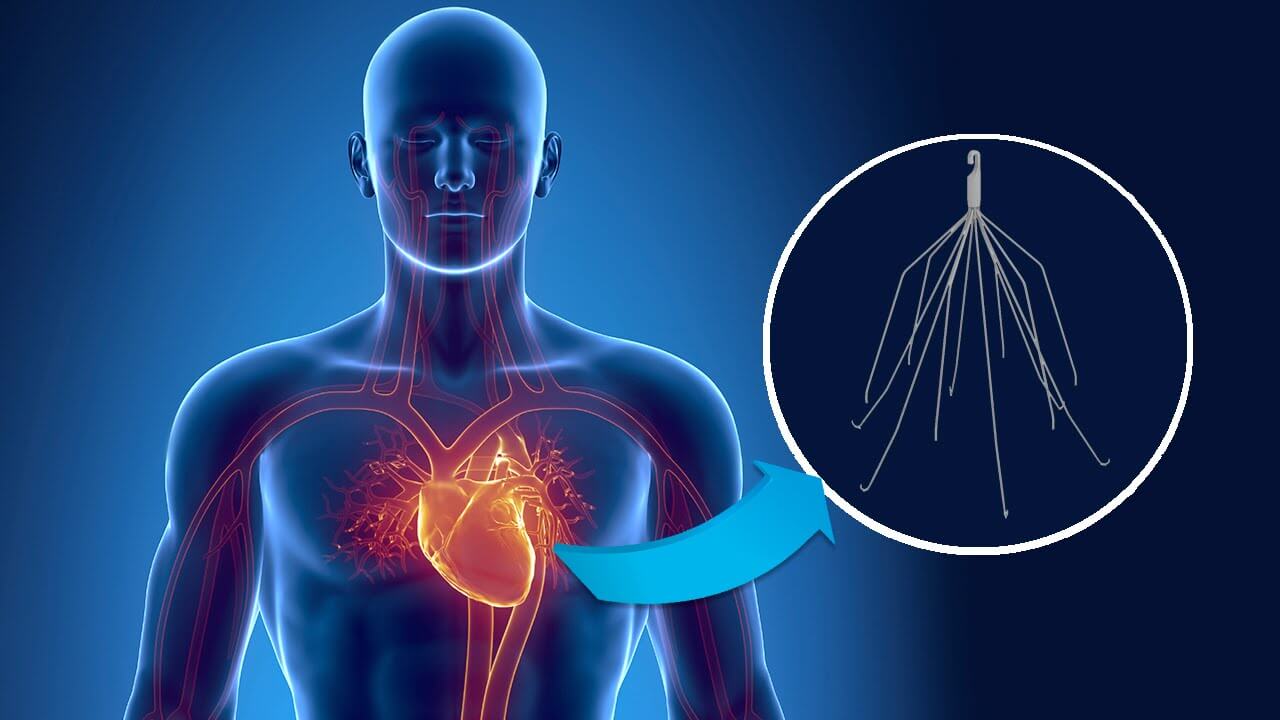
C.R. Bard faces an increasing number of lawsuits alleging its inferior vena cava filters (IVC filters) are defective and the company failed to warn patients and doctors of the risks. Among these lawsuits is one recently filed by an Oklahoma woman. The woman had a Bard Recovery filter implanted in 2003, but shortly thereafter experienced complications from the device.
The FDA approved the Bard Recovery IVC filter via the controversial 510(k) approvals process. This means the device did not undergo stringent clinical trials before it was allowed onto the market. The device was approved in 2003, and just two short years later, Bard issued a voluntary recall of its Recovery filter.
Just one year after Bard released the Recovery filter onto the market, the company began to receive complaints that the legs or struts of the device were prone to breaking off and traveling to other parts of the body like the intestines, heart, and lungs. With studies showing the Recovery’s fracture rate as high as 34%, the company decided to recall the device in 2005.
But the Recovery filter isn’t the only defective IVC filter released to the market. Other filters manufactured by Cook Medical, Cordis Corporation, and Rex Medical also have high rates of failure.
After receiving nearly 1,000 reports of defective IVC filters, the FDA issued a warning in 2010 to doctors and patients advising filters be removed as soon as the threat of blood clots has passed, because the risk of experiencing device failure increases with the amount of time the device remains in the body. In 2014 the FDA updated its warning, advising filters be removed between 24 and 59 days after implantation.
The first IVC filter lawsuits to go to trial will be against Cook Medical in early 2017. The trials could have a profound impact on other IVC filter lawsuits filed against more than half a dozen manufacturers.