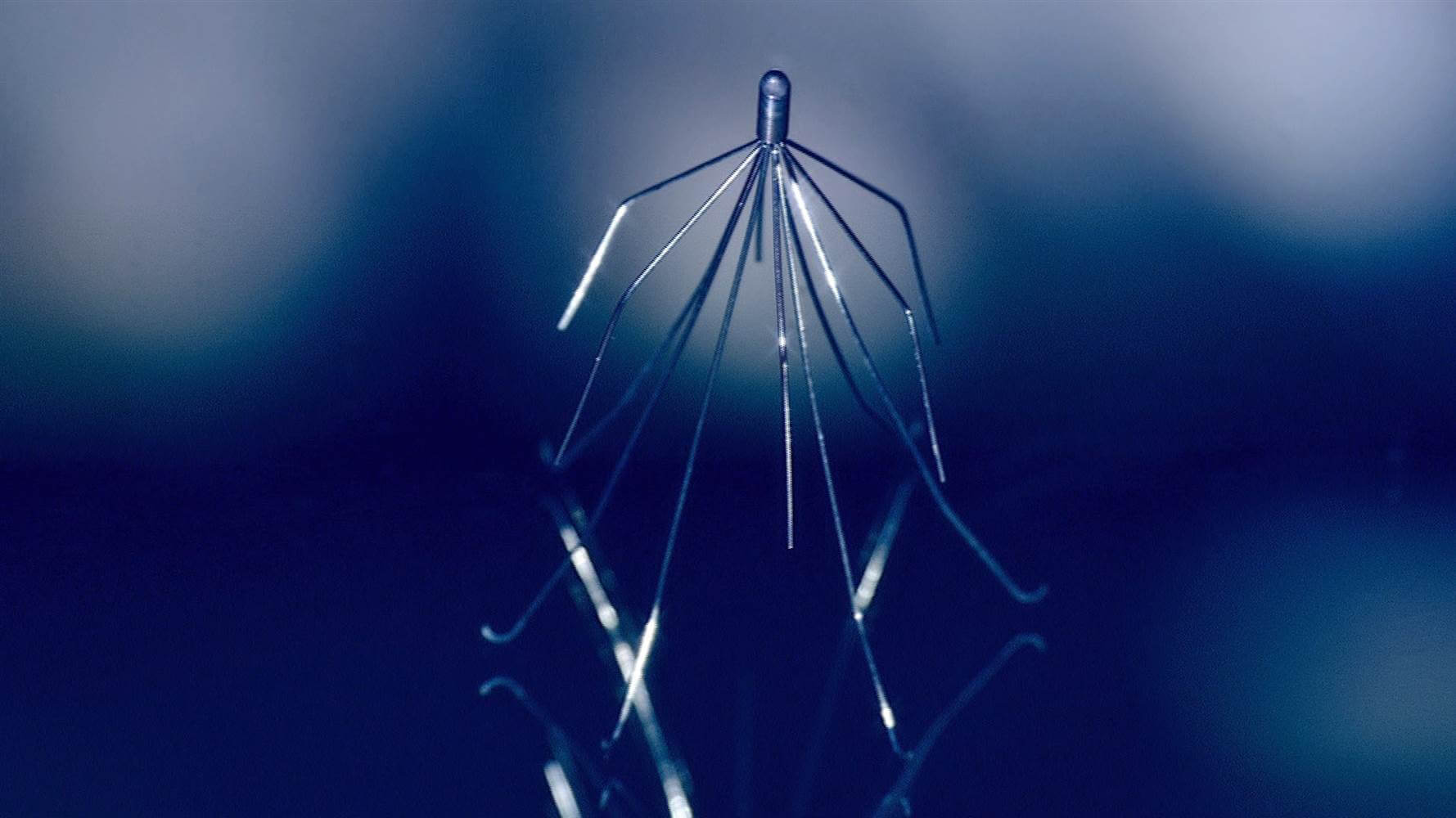
A woman recently filed a wrongful death lawsuit against C.R. Bard concerning the company’s Inferior Vena Cava filters (IVC filters). The woman’s husband had one of Bard’s G2 IVC filters implanted in April 2006 to help manage his risk of pulmonary embolism (PE), when a blood clot from the lower extremities travels into the lungs.
Shortly after implantation, the man’s IVC filter tilted and perforated his inferior vena cava vein. Because the filter tilted, it was no longer able to catch blood clots traveling from the lower extremities, and only five months after implantation the man died from a blood clot.
As tragic as this story is, it is by no means the only one. The woman now joins hundreds of other plaintiffs seeking justice for suffering similar complications from defective IVC filters.
Between 2005 and 2010, the Food and Drug Administration (FDA) received 921 reports of device failure of IVC filters. Of these reports, 328 involved device migration, 146 involved device embolization, 70 involved perforation of the inferior vena cava wall, and 56 involved filter fracture.
Bard isn’t the only medical device manufacturer facing IVC filter lawsuits either. Cook Medical, Cordis Corporation, and Boston Scientific all face lawsuits both in the United States and in Canada.
The first of these companies to go to court over the IVC filter lawsuits is Cook Medical. Although Cook was negotiating in a settlement conference in early July, the two parties could not reach an agreement. Instead, Cook will face these lawsuits during a trial set to begin this September.
The outcome of the trial could help predict how the other manufacturers will fare in their lawsuits, and Bard will only have several months before its IVC filter lawsuits come to trial in early 2017.