Last week, pharmaceutical giant Johnson & Johnson announced that it will partner with Hewlett-Packard to develop 3D printed medical devices. This is one of over 50 collaborations with other companies, government agencies, and academics to expand 3D printing medical technology.
J&J and HP are working on a 3D printed contact lens for patients who suffer from presbyopia, or farsightedness commonly caused by old age. But this isn’t the only device J&J is working on. Partnering with the Stryker Corporation and Smith & Nephew, J&J is working to develop 3D printed orthopedic implants.
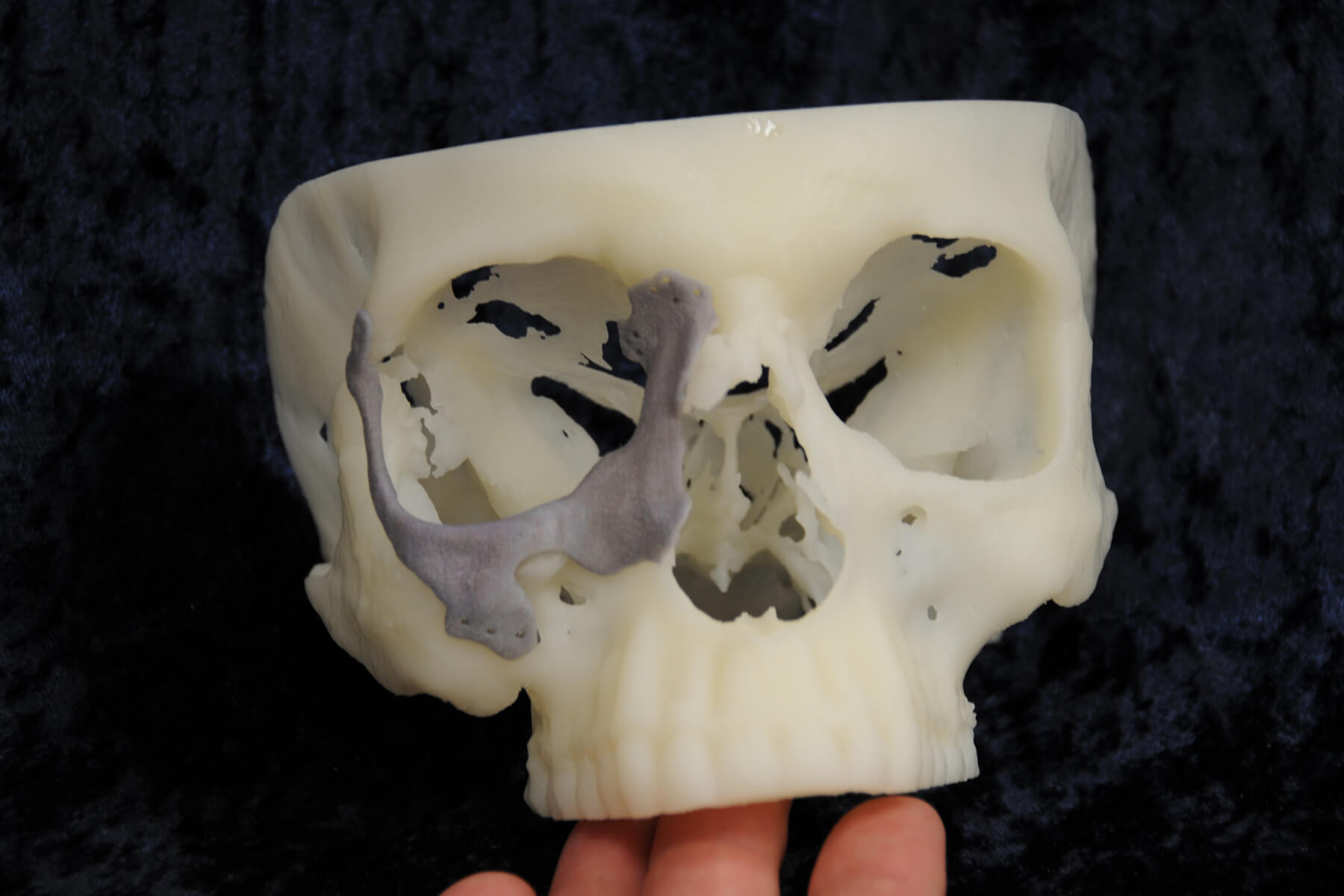
What might be J&J’s most impressive 3D printed device is a bio-resolvable, patient specific, bone graft cage. While this device could have monumental implications for the medical device industry, it also raises significant questions about safety standards. If each device is designed and printed uniquely for individual patients, how will the FDA be able to ensure the safety of each device?
The FDA recently released draft guidelines for 3D printed medical devices to help manufacturers understand how the FDA will assess them. The guidelines provide a broad overview of what aspects of the devices need to be thoroughly tested including design, sanitation, and materials. But as 3D printing technology continues to advance, the FDA might have to reconsider the specifics of 3D printed medical devices.