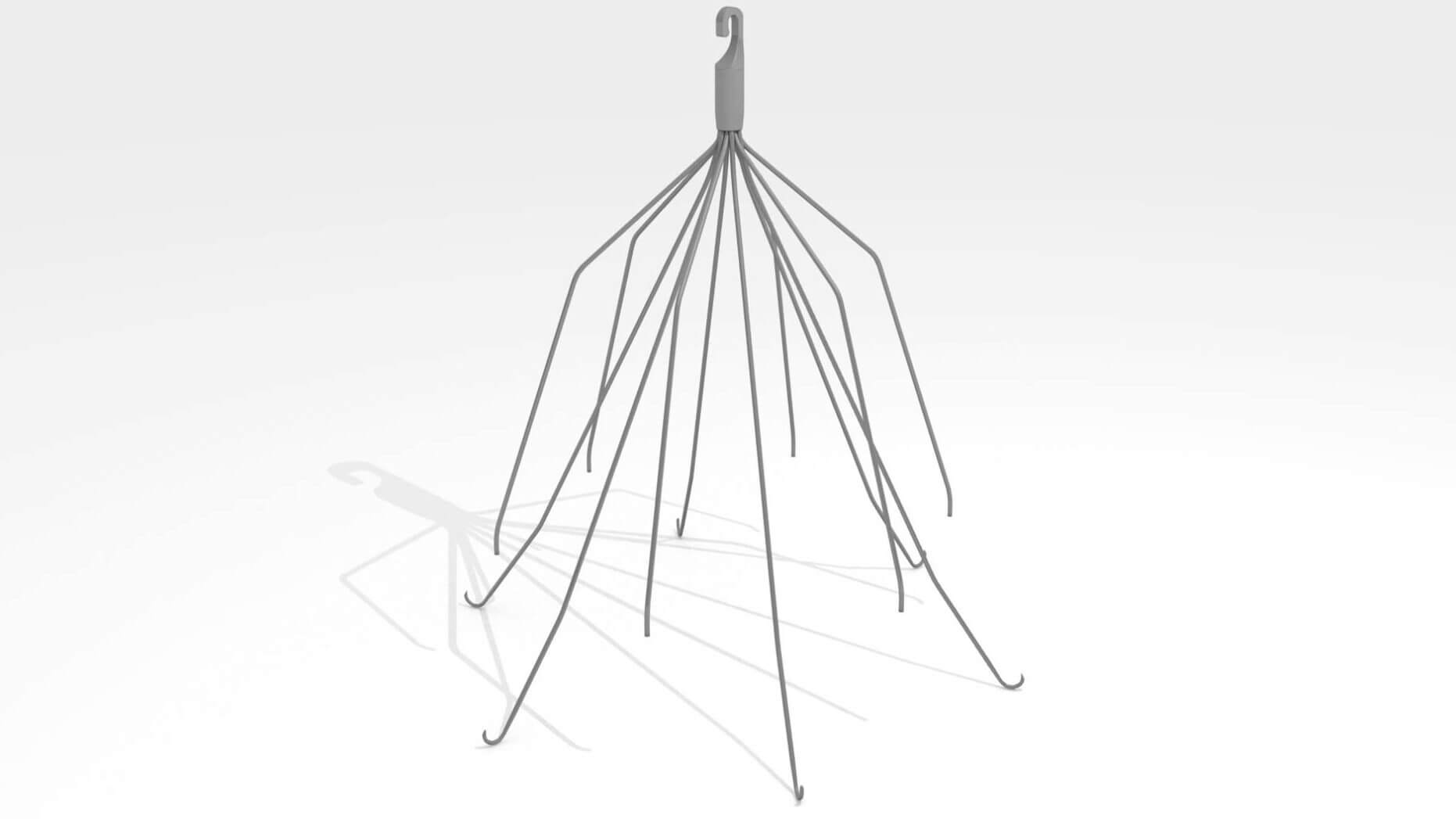
Cook Medical and plaintiffs in the Inferior Vena Cava Filter (IVC Filter) lawsuits have finally agreed on case selections for bellwether trials, which will begin in September. Over 400 lawsuits have been filed against Cook regarding its Celect and Günter Tulip IVC Filters that caused serious injuries.
The lawsuits allege that Cook failed to warn doctors and patients of the increased risks of migration, fracture, embolization, and perforation of the device. Studies have shown that Cook’s IVC Filters have an extremely high rate of perforation, as much as 86% from a study published in Cardiovascular and Interventional Radiology back in 2012.
Between 2005 and 2010, the Food and Drug Administration (FDA) received over 900 reports of adverse events caused by IVC Filters, prompting the federal agency to issue a warning statement to doctors and patients recommending the device only remain in the body between 39 and 54 days as the risk of adverse events increases with time.
IVC Filters are used to catch blood clots to prevent them from traveling into the heart and lungs while still allowing blood to flow through the vein. The medical devices are most commonly used in patients who have sustained traumatic injuries or have undergone surgery, thus putting them at an increased risk of developing blood clots.
Cook is only one of several manufacturers being sued regarding defective inferior vena cava filters. C.R. Bard and Cordis are also facing hundred of lawsuits regarding the safety of these devices.